Abstract
Introduction
DLBCL is a commonly diagnosed, aggressive non-Hodgkin's lymphoma with ~40% of patients experiencing refractory or relapsed disease. Development of alternative therapies that target molecular features defining these unresponsive tumors is an active area of research to significantly advance the field and improve these patient's clinical management. However, few DLBCL animal models exist to test the efficacy of newly developed treatments, and are restricted to transgenic or xenograft mice that often fail to recapitulate the heterogeneous sub-classifications of this complex disease. While transgenic mice allow for spontaneous tumor formation, these models rely on inducing expression of specific oncogenes that drive a select group of DLBCL. The xenograft model offers several advantages, such as reproducing late-stage disease and shortening the model development time, but consist of implanting the tumor cells in a localized region or subcutaneously into immune-deficient mice. Despite some benefits of the transplant approach, these models are limited by their engraftment reproducibility and interactions with host micro-environments. Here, we explored the utility of humanizing Nod-Scid-IL2Rg null (NSG) mouse strains with factors associated with enhancing myeloid and lymphoma cell growth to establish a pipeline for rapid, reliable generation of in vivo DLBCL models.
Methods
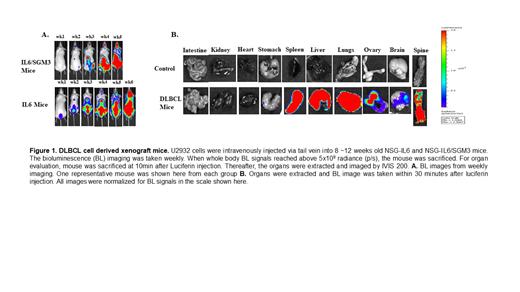
We transduced the well-established human DLBCL cells, U2932, with the luciferase (Luc)-EGFP gene. The Luc-expressing (U2932-Luc) tumor cells were sorted for GFP positivity (GFP +) and expanded. The U2932-Luc cells (1 x 10 6/100µl PBS) were injected IV via tail vein into 8~12-week-old mice of various humanized NSG strains (representing equal numbers of each sex). NSG mice were humanized by transgenic expression of human cytokines (either human IL6 alone or IL6 plus SCF, GM-CSF, and IL-3) with the CMV promoter. Each experiment included of U2932-Luc cell transplanted group and control groups. We assessed U2932-Luc cell engraftment and growth by weekly in vivo imaging (IVIS 200 Imager, Perkin Elmer). To evaluate the organ specific engraftment/progression, we confirmed engraftment by bioluminescence imaging at the 2 nd week, then euthanized one mouse per week. At 15 minutes before euthansia, luciferin was injected via intraperitoneal injection. Following euthanasia, the organs were excised and underwent ex vivo IVIS imaging. The spleen, lungs, and liver were then fixed with 10% formalin and embedded in paraffin. Sections were stained with hematoxylin and eosin, and an anti-CD20 antibody to evaluate the tumor morphology using a Zeiss AXIO Imager M2 microscope (Zeiss, Nashville, TN). All other mice were monitored for survival and the median survival between the IL6 and IL6/SGM3 mice were compared using the Log-rank test.
Results
Similar to previously reported DLBCL humanized strain (MISTRG) (Hashwah, 2019), we used the IL6/SGM3 expressing strain. However, our studies also included the IL6 only humanized strain. We found that both the IL6 and IL6/SGM3 strains were highly permissive to DLBCL growth. The IL-6 strain exhibited a heightened growth of U2932 cells relative to the IL-6/SGM3 mice. As shown in Figure 1, the IL6 mice survived longer than IL6/SGM3 mice. Significant difference between the median survival of IL6 and IL6/SGM3 mice i.e. 48 days vs 42 days was observed (p < 0.0482). The organ specific evaluation demonstrated that U2932-Luc cells were initially engrafted and grew in the lung, liver, and spleen. Subsequently, U2932 cells were found in the skeleton, ovary, and brain. Of note, we detected significantly enlargements of the kidney, spleen, and ovary at the terminal stage.
Conclusions
Our humanized mouse model approach of using U2932 human DLBCL cells transduced with the Luc gene in the NSG-IL6 and NSG-IL6/SGM3 mice reproduced the clinical features of an aggressive DLBCL that paralleled the original patient. This model will provide a new tool to enable expansion of patient samples while overcoming the current limitations of DLBCL xenografts and transgenic mice. The ability to maintain growth of patient-derived samples within clinically relevant locations has great potential to more accurately test patient-specific, personalized treatment strategies.
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal